Hungary’s new poisons notifications cost

ADACT’s advice has always been to only notify in the countries where you plan to sell your products for simplicity. Now there is another reason to do this, with the first countries requiring additional payment for processing Poison Centre Notifications (PCNs) in their own domestic market. The European Chemicals Agency (ECHA) have advocated a minimal […]
MHRA invoicing is changing…for the better
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) now requires payment to be made at the time of TPD submission. The benefit being that your Tobacco Products Directive (TPD) submissions will be reviewed and placed on the positive list much sooner. Previously you may have had to wait for your invoice from the MHRA, […]
Warning: Fake tobacco free nicotine is out there
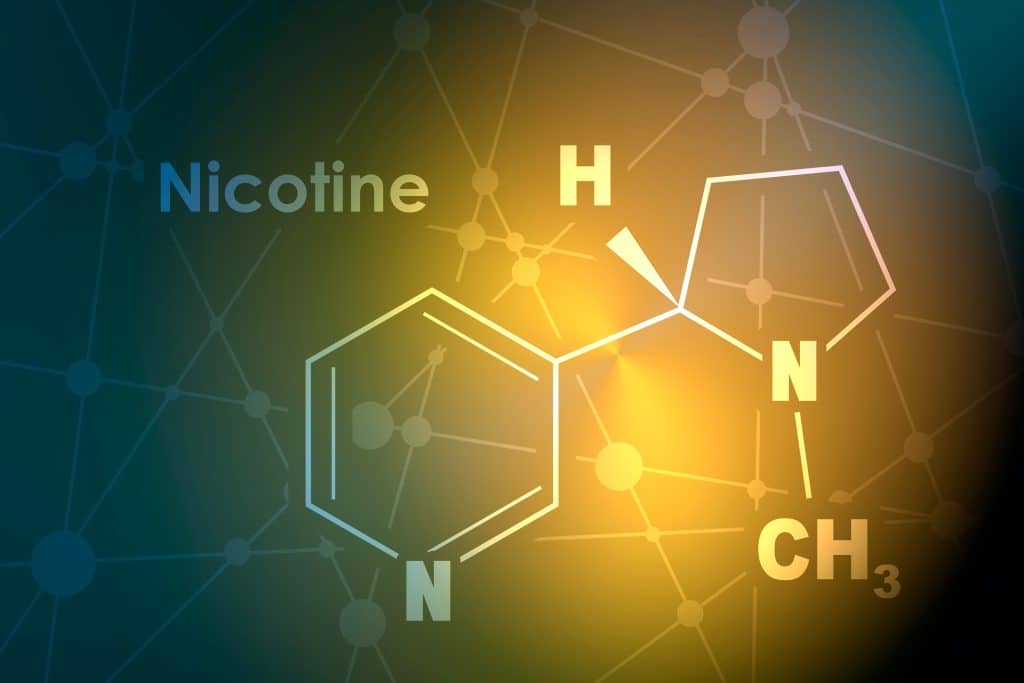
Following recent regulatory events in the USA, many companies are moving to synthetic or tobacco free nicotine in their products, but most is fake. Some products that we have tested have been tobacco derived nicotine labelled as synthetic. If challenged, can you prove you are using synthetic nicotine? ADACT can help! We are able to […]
The latest on PMTA
Following the most recent Food and Drug Administration (FDA) presentation on the progress of Premarket Tobacco Product Application (PMTA) review, our Chief Regulatory Officer shares some of the key details along with how we think this will impact vape products in the market. Some of the key details from our Chief Regulatory Officer, Damien Bové, […]
German e-liquid tax coming in 2022

On 11 June the Germany Bundestag approved legislation to make smoking more expensive as of next year with an added tax. The greatest increases have been reserved for e-cigarettes and tobacco-heating products, which were previously only lightly taxes. Currently, a 10-millilitre bottle of vape liquid costs around €5 in Germany. In 2022, an extra €1.60 […]
Laboratory excellence

The ADACT team are proud to announce that on 9 April 2021 our laboratory received ISO/IEC 17025:2017 accreditation. Following a lengthly period of assessment, Perry Johnson Laboratory Accreditation, Inc. has awarded the accreditation which demonstrates our technical excellence in the Chemical Testing of e-liquids and e-devices and measuring Harmful and Potentially Harmful Constituents (HPHCs). ADACT’s […]
Changes to vaping in New Zealand

After a successful launch, the rollout of New Zealand’s Smokefree Environments and Regulated Products (Vaping) Amendment Act is well underway with further changes coming into force on 11 May 2021. The Act which passed on 11 August 2020 has been developed to strike a balance between ensuring vaping and electronic cigarette products are available for […]
Cosmetic products require new registration

Following the UK leaving the European Union on 1 January 2021, cosmetic products – including those with CBD or hemp based ingredients – require re-registering. If you make cosmetic products, which are available to consumers in Great Britain, you are required to notify your cosmetic products using the UK’s own Submit Cosmetic Product Notification Portal […]
Take part in a new public consultation

The Department of Health and Social Care (DHSC) is carrying out a public consultation regarding The Tobacco and Related Products Regulations 2016 (TRPR) and The Standardised Packaging of Tobacco Products Regulations 2015 (SPoT). While the UK’s tobacco control legislation is already among the most comprehensive in the world and smoking rates are at their lowest […]
New PMTA uncertainty

The U.S. Food and Drug Administration (FDA) displayed the final rule for the Premarket Tobacco Product Application (PMTA) in the federal register, but it did not get published on 19 January 2021. A day later on 20 January, a memo from The White House Chief of Staff ordered the withdrawal of any rules that did […]
