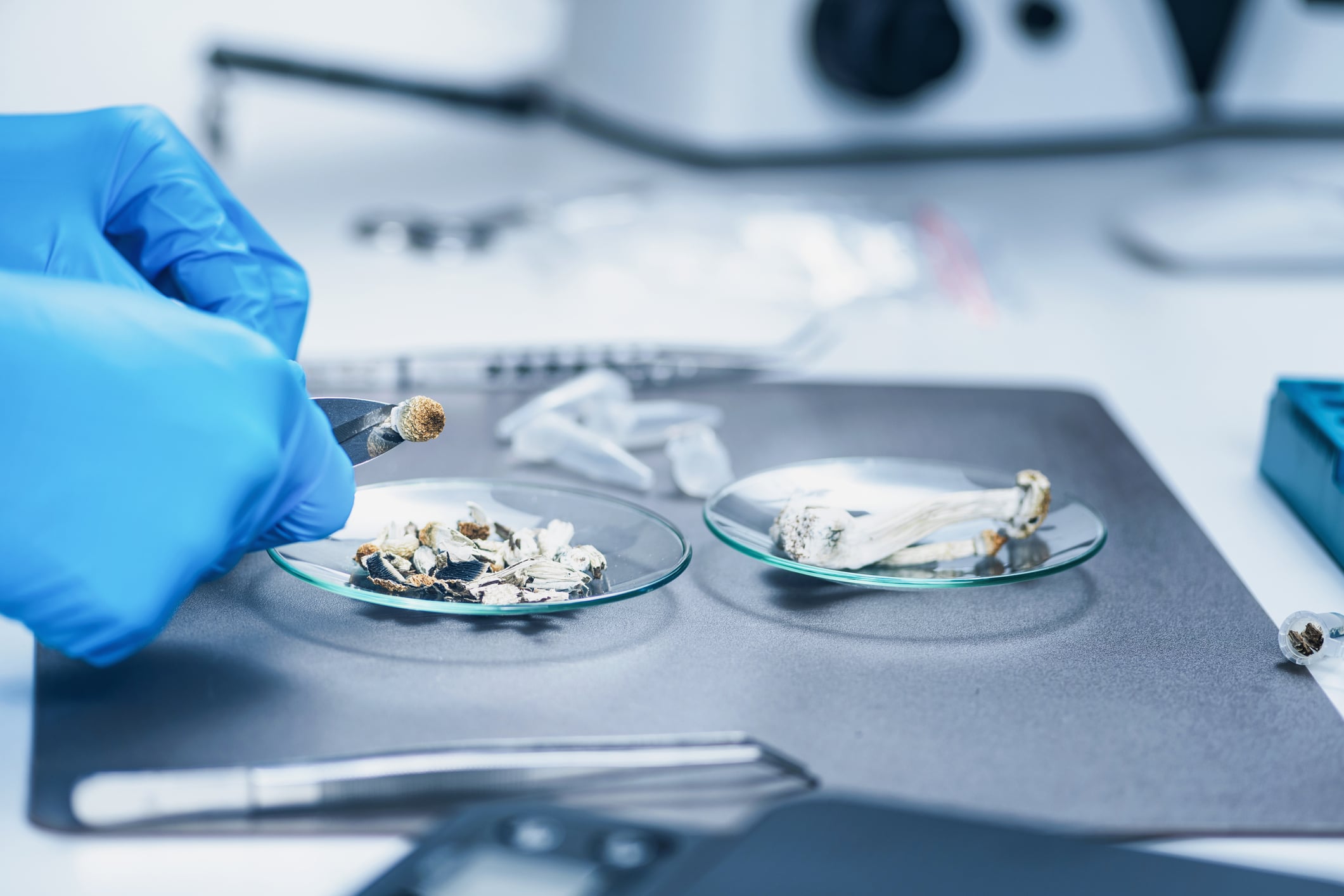
We’re delighted to announce that ADACT Medical has been granted a controlled drugs license by the Home Office for the purpose of analysing and testing a range of CBD products at our state-of-the-art laboratory in the United Kingdom.
This license allows ADACT to expand its’ existing regulatory compliance and analytical testing services for consumer cannabidiol / CBD products into areas seeing rapid and exciting developments, such as medicinal cannabis.
Please note under the conditions of the licence and the Misuse of Drugs Act 1971 you must contact ADACT to obtain written approval prior to submitting materials for testing.
To discuss our five-day cannabis testing and other services, please call +44(0)1302 986 088 or email [email protected]